Pharma: Page 27
-
How EMD Serono plans to hit its lofty goals in R&D
The company’s global heads of oncology R&D reveal how strategic partnerships are key to its launch plans following layoffs in the U.S.
By Alexandra Pecci • Nov. 27, 2023 -
As alcohol-related deaths climb, new drugs could curb the urge to drink
A drug developed by Kinnov Therapeutics halved alcohol consumption in heavy drinkers in a mid-stage study.
By Kelly Bilodeau • Nov. 27, 2023 -
 Explore the Trendline➔
Explore the Trendline➔
 Getty Images
Getty Images Trendline
TrendlineArtificial intelligence & machine learning
After years of excited buzz around the potential of artificial intelligence and machine learning, pharma has begun to realize the true implications and potential value of these technologies.
By PharmaVoice staff -
The biopharma COO’s playbook
Connie Chang, COO at ONL Therapeutics, describes what it takes to bring a CEO’s vision to life.
By Alexandra Pecci • Nov. 21, 2023 -
Biohaven wants to muscle in on the Ozempic craze with a competing class of drugs
Biohaven and other companies are aiming to develop meds with a different mechanism of action that trim the waistline while building lean tissue.
By Kelly Bilodeau • Nov. 20, 2023 -
A startling drop in U.S. life expectancy — and how pharma could help turn the tide
The recently observed dip was particularly acute for men, who now live an average of 5.8 fewer years than women.
By Meagan Parrish • Nov. 17, 2023 -
Q&A
AstraZeneca showed the pandemic isn’t over for immunocompromised patients. What’s next?
Dr. Paul Moss, a hematology expert in the U.K., conducted a study with AstraZeneca showing the alarming extent to which immunocompromised patients are burdened with COVID-19.
By Michael Gibney • Nov. 16, 2023 -
The power of genomics-based healthcare remains largely untapped
23andMe co-founder and CEO Anne Wojcicki discusses ways the industry could integrate genomics into healthcare.
By Alexandra Pecci • Nov. 15, 2023 -
6 ways to improve clinical trial diversity — as shown by J&J
Johnson & Johnson Innovative Medicine’s director of diversity, equity and inclusion in clinical trials shares key strategies for making DEI work.
By Alexandra Pecci • Nov. 14, 2023 -
Humans can’t shake the mosquito threat. Here’s what pharma has in the pipeline.
The arsenal against the world’s deadliest creature is growing and a universal vaccine is in the works.
By Kelly Bilodeau • Nov. 13, 2023 -


stock.adobe.com/master1305
 Sponsored by ClinicalMind
Sponsored by ClinicalMindOptimizing KOL engagement and technology utilization in 2024
What if your advisory solution was a skillful blend of innovative tech, medical communications specialists, high-touch client service, and a flexible contract?
Nov. 13, 2023 -
5 of the best paid biopharma CEOs
As revenue growth remained high last year, so did biopharma CEO pay in the U.S.
By Alexandra Pecci • Nov. 10, 2023 -
How pharma leaders talk about ethics in a highly criticized industry
The maze of ethical issues in biopharma can confound even the wiliest executive. How do some leaders get by?
By Michael Gibney • Nov. 9, 2023 -
An at-home flu vaccine? If approved, it could open the door to more DIY options
A new way to administer vaccines at home could help AstraZeneca reach a wider market for FluMist, the nasal influenza vaccine.
By Kelly Bilodeau • Nov. 8, 2023 -
The obesity drug effect: What medical device executives are saying
Investor concerns that interest in GLP-1s will slow demand for medical devices and procedures have shaved roughly $370 billion in value from stocks across the medtech sector, according to research by Mizuho.
By Susan Kelly • Nov. 6, 2023 -
Opinion
The thorniest questions facing pharma, according to a leading bioethicist
Bioethics guru Arthur Caplan of NYU gives his outspoken and frank opinion on the industry’s minefield of ethical challenges.
By Michael Gibney • Nov. 6, 2023 -
PharmaVoice 100
PharmaVoice 100s: Entrepreneurs
From biotech startups to a services agency, these entrepreneurs are hedging their bets to offer innovative approaches in patient care.
By Meagan Parrish • Nov. 3, 2023 -
How Big Pharma keeps the oncology engines hot with a host of strategies
From Merck and J&J to AbbVie and Eisai, the oncology field has stayed consistently devoted to churning out important therapies.
By Michael Gibney • Nov. 2, 2023 -
Tools like ChatGPT can personalize clinical trials tech — if companies avoid the pitfalls
From hallucinations to data privacy, pharma looks to iron out the kinks of generative AI.
By Kelly Bilodeau • Nov. 1, 2023 -
Roivant’s $7B Roche deal was a ‘moment of opportunity,’ CEO says
Roivant Sciences CEO Matt Gline and Telavant CEO Frank Torti know Roivant’s position as buyers and sellers in the pharma industry, and they’re embracing those opportunities.
By Michael Gibney • Oct. 31, 2023 -
Q&A // First 90 Days
Wisp’s ‘bold and unapologetic approach’ to women’s sexual and reproductive health
In the role of interim CEO of Wisp, Monica Cepak aims to break down barriers and expand access via telehealth.
By Alexandra Pecci • Oct. 31, 2023 -
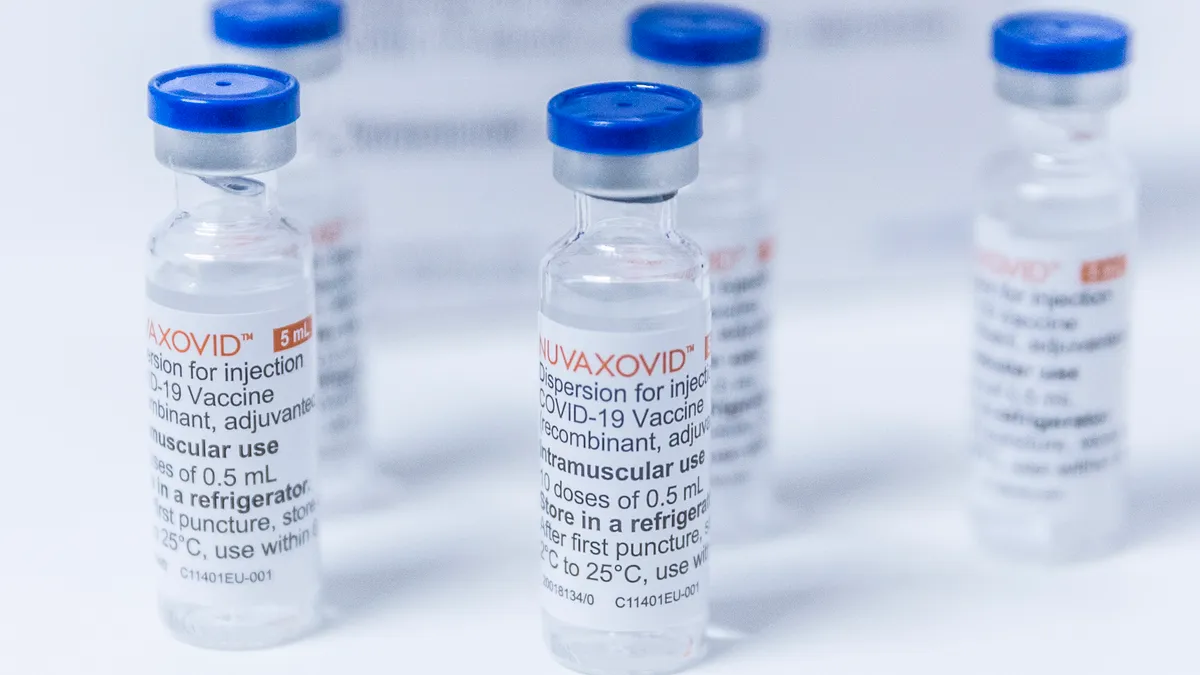
What’s next for COVID dark horse Novavax?
Novavax scored a new COVID vaccine go-ahead for the 2023-24 season, but is it the boost the company needs?
By Kelly Bilodeau • Oct. 30, 2023 -
Q&A // Red Jacket winners 2023
Red Jacket: Dr. Mark Goldberg, a clinical innovator
Dr. Mark Goldberg has continually been at the cutting edge of technologies and ideas, including now as chairman and CEO of Allucent.
By Alexandra Pecci • Oct. 27, 2023 -
The 2023 Red Jacket honorees
This year's inductees to the PharmaVoice 100 "hall of fame" are leaders who have been at the forefront — and will stay at the forefront — of industry change.
By Meagan Parrish • Oct. 27, 2023 -
Q&A // Red Jacket winners 2023
Red Jacket: Dr. Jeremy Levin, a transformational leader
A consummate life sciences executive who has led with boldness and devotion, Dr. Jeremy Levin considers himself a participant as well as a leader.
By Michael Gibney • Oct. 27, 2023 -
Q&A // Red Jacket winners 2023
Red Jacket: Helen Sabzevari, an immunotherapy pioneer
Precigen’s CEO is pushing for her next therapeutic breakthrough with innovative cell and gene therapy platforms.
By Meagan Parrish • Oct. 27, 2023