Pharma: Page 22
-
As drug shortages reach record highs, regulators float next steps
With many chemo and ADHD drugs stuck in stubborn shortages, several agencies are looking for new solutions to boost supplies.
By Kelly Bilodeau • May 6, 2024 -
Takeover target? Activist investor believes Novavax tech would be in ‘better hands’ with Big Pharma
With its next earnings report looming, pushback from an investor is adding pressure on Novavax to boost performance.
By Meagan Parrish • May 3, 2024 -
 Explore the Trendline➔
Explore the Trendline➔
 Getty Images
Getty Images Trendline
TrendlineArtificial intelligence & machine learning
After years of excited buzz around the potential of artificial intelligence and machine learning, pharma has begun to realize the true implications and potential value of these technologies.
By PharmaVoice staff -
$4M+ gene therapies? How payers can adapt to a new reality of pricey ‘cures’
Pricey gene therapies promise great benefit to patients but pose a threat to the payer landscape — ICER and a Tufts think tank are offering potential solutions.
By Michael Gibney • May 2, 2024 -
As AI proliferates in pharma, regulators look to catch up in clinical trials
The FDA’s newly launched Center for Clinical Trial Innovation potentially opens the door for more efficient and expanded AI use, but leaves pharmas wanting more guidance.
By Amy Baxter • May 1, 2024 -
Profile
Now a biopharma powerhouse, Vertex’s R&D bedrock could deliver tomorrow’s blockbusters
From its landmark CRISPR approval to its potential breakthrough drug for pain, Vertex’s development approach, led by chief scientific officer Dr. David Altshuler, is paying off.
By Michael Gibney • April 30, 2024 -
As BMS announces major cuts, its Karuna deal looks poised to drive growth
The company's schizophrenia drug picked up in its $14 billion acquisition of Karuna Therapeutics recently generated positive long-term data from its phase 3 program.
By Kelly Bilodeau • April 29, 2024 -
Q&A
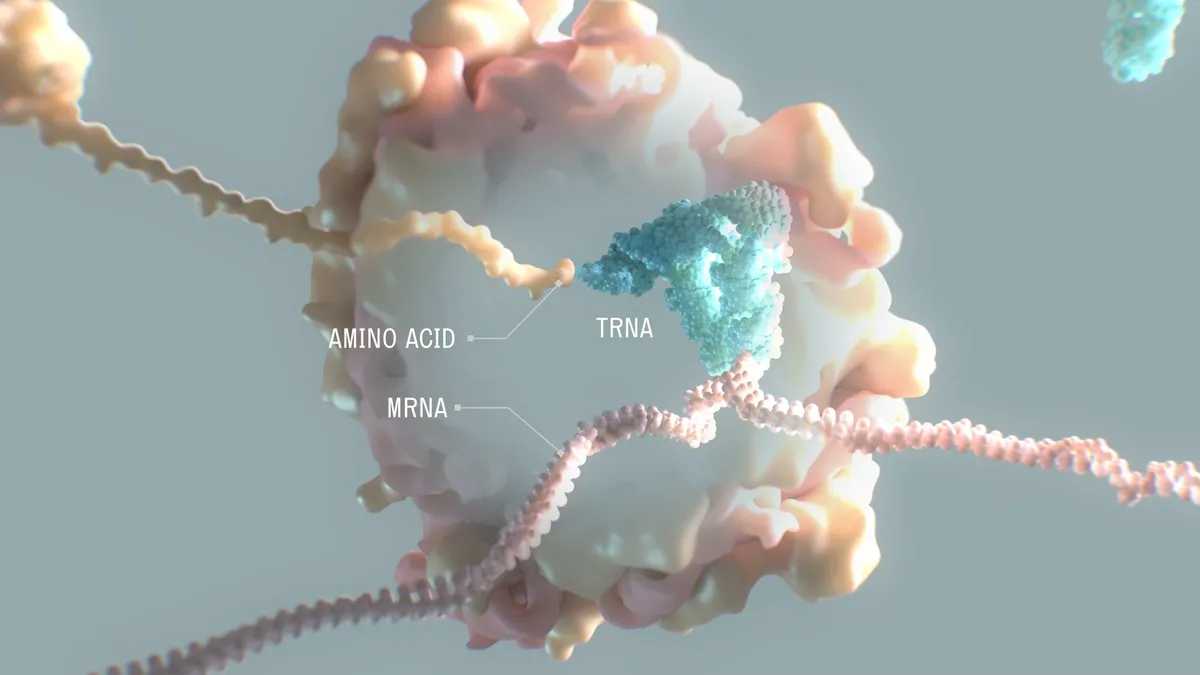
How a biotech exec is leveraging Big Pharma lessons to advance tRNA
Michelle Werner shares how the lessons from her resume — and personal experience — are driving her work with novel therapeutics.
By Amy Baxter • April 29, 2024 -
As Lilly and BMS invest in manufacturing, demand for drugs still outweighs supply
From popular weight loss drugs to lifesaving cancer therapies, recent pharma deals show the industry is doubling down on manufacturing capacity to meet demand.
By Michael Gibney • April 25, 2024 -
Biogen: Don’t expect any big acquisitions this year
While the company is eager to diversify, CEO Chris Viehbacher said that in the near-term any dealmaking would likely focus on collaborations and early-stage assets.
By Jacob Bell • April 24, 2024 -
Q&A
AbbVie and Regenxbio’s ‘multibillion-dollar opportunity’ in vision loss gene therapy
After a successful mid-stage study, the companies aim to upend traditional wet AMD treatments with a one-time gene therapy.
By Amy Baxter • April 24, 2024 -
Inside J&J’s strategy to de-gender clinical trials
Mark Wildgust, vice president of global medical affairs for J&J's oncology division, shares strategies for making clinical trials gender- and trans-inclusive.
By Alexandra Pecci • April 23, 2024 -
Behind the ‘encouraging’ new results for Amylyx’s beleaguered drug
Where Amylyx ultimately failed in ALS, it could prevail in a rare disease. The doctor leading this research explains why.
By Meagan Parrish • April 19, 2024 -
Roivant spinoff tackles growing complexity of clinical trials
As precision medicine booms, all trials are starting to look like rare disease trials, the company said.
By Kelly Bilodeau • April 18, 2024 -
3 patent expirations in 2024 and how companies are pivoting
As the cliffs approach, pharma companies are tackling the sales hit with diverging strategies.
By Amy Baxter • April 17, 2024 -
Deep Dive
Ukraine was pharma’s ‘darling’ of clinical trials. As war drags on, will the industry come back?
New clinical trial starts are picking back up, but are still far below their bustling pre-war level.
By Meagan Parrish • April 16, 2024 -
Where the GLP-1 weight loss market goes will depend on data
As GLP-1s expand into new disease categories, their impact could be enough to overtake leading cardiovascular drugs.
By Amy Baxter • April 15, 2024 -
3 big recent trial flops
How these clinical setbacks impacted the companies, industry and patients.
By Meagan Parrish • April 12, 2024 -
Q&A
How pharma’s surging M&A is impacting the industry’s tech sector
As dealmaking in biopharma picks up, the CEO of eClinical Solutions sees an opportunity for clinical software companies to make acquisitions easier to close.
By Michael Gibney • April 11, 2024 -
Biotech Spotlight
What’s old is new: The revival of a one-time radiotherapy cancer treatment
TAE Life Sciences is bringing radiation-based cancer therapy to clinical trials globally in the coming years.
By Amy Baxter • April 10, 2024 -
Deep Dive
Psychiatry drugs finally have pharma’s attention. Can they keep it?
Recent biotech company acquisitions have put emerging schizophrenia treatments in focus. But many development hurdles still stand in the way of new medicines for the brain.
By Jacob Bell • April 10, 2024 -
Biosimilar uptake appears to finally be on the upswing, and Biocon Biologics is betting on the sector’s future
Biosimilars promise market competition for branded biologics, which in theory could drive down prices. But so far, winning market share has been a struggle.
By Michael Gibney • April 9, 2024 -
Cutting pills. Rationing insulin. How Americans struggle to pay for drugs.
More than 20% of Americans are forgoing prescription medications due to costs, according to a recent survey.
By Amy Baxter • April 9, 2024 -
Women want to participate in clinical trials. Lack of flexibility is still a problem.
Underrepresentation of women in clinical trials affects the resulting drugs that are available down the line, but even small changes can fuel participation.
By Kelly Bilodeau • April 8, 2024 -
Next-gen menopause treatments have blockbuster potential. Can women, doctors and payers be convinced?
The market is huge, but will companies overcome the triple hurdles of payer hesitance, prescriber reluctance and sluggish consumer demand?
By Alexandra Pecci • April 8, 2024 -
Sponsored by Avenga
Unlocking growth: How GenAI can help pharma companies convert new clients
GenAI is profoundly reshaping various facets of the pharmaceutical industry. Projections indicate that the global GenAI market in pharma will soar to $2258.1 million by 2032.
By Olena Domanska, Data Science Engineering Manager at Avenga • April 8, 2024